How to reduce nox emissions
How to reduce nox emissions
NOx Emission
Thus, NOx emission in the systems of the described structure is a weighted average of the quantity of oxides produced during the combustion of pulverized fuel of high (CM – concentrated mixture) and of low concentration (DM – diluted mixture).
Related terms:
Download as PDF
About this page
Assessment of performance, combustion, and emission behavior of novel annona biodiesel-operated diesel engine
14.3.4.3 NOx emission
NOx emission is found in an engine due to higher in-cylinder temperature. Though the diesel fuel or the ester-based biodiesel does not contain nitrogen, the NOx are formed by taking the nitrogen from the air because 78% of nitrogen is present in the air.
Fig. 14.7 shows the variation of NOx emission with brake power for different proportions of AME diesel blends and diesel. It is noted that the NOx increases with increasing brake power for all AME-diesel blends at all loads. Among the different AME-diesel blends, A20 has a lower NOx emission than that of other blends. The NOx for A20 is 620 ppm and for diesel is 552 ppm at maximum load. The diesel fuel has less NOx emission because the diesel fuel contains no oxygen content. When the biodiesel is present in the combustion chamber, the temperature becomes very high due to the presence of higher oxygen content, which enhances combustion and results in higher NOx emission.
Integrated Gasification Combined-Cycle Power Plants
I.G NOx Emissions Control
NOx emissions from the combustion turbine can result from two sources: (a) fuel-bound nitrogen in such species as ammonia and (b) thermally produced NOx. In most of the commercial-size IGCC plants there is a water wash step in the cold gas cleanup that removes ammonia and HCN from the syngas before entering the AGR process, thereby removing the components of fuel-bound nitrogen.
Due to its high flame temperature, the clean syngas can lead to high NOx emissions in the combustion turbine unless controlled by other means. Two main techniques are used to lower the flame temperature for NOx control in IGCC systems. One is to saturate the syngas with hot water derived from low-temperature heat recovery elsewhere in the process. The other is to use nitrogen from the ASU. In both cases, mass is also added to the syngas and additional power is thereby generated in the gas turbine and steam cycle. At Wabash NOx control is by saturation and some steam injection. At Tampa the NOx is controlled by nitrogen injection, while at Buggenum and Puertollano a combination of saturation and nitrogen is used.
At the Sierra Pacific project the nitrogen in the low-heating-value syngas from the air-blown gasifier should reduce the flame temperature sufficiently to meet the NOx limits. However, this has not yet been demonstrated and there may also be some NOx production from ammonia at this plant since there is no water wash step in the gas cleanup. If extremely low NOx levels, such as 1–2 ppmv, are required, a selective catalytic reduction (SCR) bed can be placed within the HRSG at a suitable temperature.
Air Pollution from Small Two-Stroke Engines and Technologies to Control It
13.1.1 NOx Emissions
NOx emissions are mainly composed of NO molecules and a small amount of NO2, in a typical ratio of 1 to 100. NO is one of the intermediate products of chemical reactions involving nitrogen and oxygen atoms and molecules, which occur at the high-temperature burned gases behind the flame. Rapid cooling (below 1800 K), due to the expansion stroke, does not allow the products to attain chemical equilibrium and some intermediate products (including NO) “freeze” and leave NO concentrations far in excess of levels corresponding to equilibrium at exhaust conditions. The high residual fraction, which is typical to two-stroke-cycle engine, results in lower maximum temperatures and therefore lower NO production.
The strong effect of the temperature is evident; a reduction of the maximum temperature from 2500 K to 2300 K results in a cutback of 90 percent in the initial rate of NO production. Increasing the residuals mass fraction is an effective and practical method of reducing the maximum temperature and thus the NO emission level. Due to the peculiar gas exchange process in two-stroke-cycle engines the recycled exhaust gas is an inherent property, and the typical low NO emission level of a two-stroke-cycle engine is anticipated. Owing to the exceptionally high value of water-latent heat, introducing water spray into the induction manifold during the cylinder (or crankcase) charging process is another practical method to depress the adiabatic flame temperature and, hence, the NO emission level.
Clean Coal Technologies for Advanced Power Generation
Limestone Effects
Reduction of nitrogen oxide emissions
4.3.5 Flue gas recirculation
In the case of the PF boilers, with relatively large furnaces (volumetric heat release qv ≈ 100–120 kW/m 3 ) the introduction of flue gas recirculation into the combustion area (directly, via suitable nozzles, via pulverizers or mixing secondary air with flue gas) complicates the technical solutions substantially and fails to produce significant effects of reducing NOx emission. This is because the reducing effect of recirculation consists mainly of the lowering of temperatures in the furnace so that the secondary formation of nitrogen oxides in its top part does not happen. In small furnaces, with a high qv, the furnace outlet temperatures are above 1400 o C, and the effect of recirculation is more visible.
Introduction
9.3.1 Chemical Thermodynamics
The crucial task for lowering diesel emissions is to improve particulate and NOx emissions simultaneously—a difficult task, as there is usually a trade-off between these two emissions. To determine if this task is at all possible, basic fundamentals of chemical thermodynamics must be explored.
Figure 9.9 shows a typical mixture distribution in a diesel injection spray just prior to ignition, ranging from rich in the spray core to lean in the spray edge. The diagram on the left shows the temperature history of the zones with different relative air-fuel ratios (λ), proceeding from the curve of mixture temperature before combustion up to the curve of burned gases after combustion. The diagram also shows the zones of soot and NOx formation, the darker shadowed zones indicating higher formation rates. It can be seen that in an indicated target λ-range, combustion is possible without soot formation and only very low NOx formation. Thus, in principle, smokeless, low-NOx diesel combustion should be possible, if the mixture formation could be narrowed to the required mixture ratio. This may not be possible with the combustion of typical sprays. However, even if soot formation cannot be completely avoided, there is a possibility of burning the soot during the high-temperature combustion process, as is shown in the diagram on the right side of the figure. This, of course, requires intensive mixing at the beginning of the expansion process while the temperatures are still on a high level, although not too high because of NOx formation. In this way the soot generated can be burned nearly completely during the process.
It can be concluded that a careful mixing strategy is the key to low-emission diesel combustion. Since mixing is controlled by fuel injection as well as by air motion in the combustion chamber, both processes must be developed concurrently. Future diesel combustion development approaches follow these guidelines. Typical design features addressing this fact are discussed next.
Combustion-Related Emissions in CI Engines
10.6.2.1 Background
EGR has an effect on the flame structure, temperature, and species profiles following ignition. The reductions in NOx emissions with EGR can be explained on the basis of a predicted decrease in peak flame temperature in a diffusion flame coupled with a reduced oxygen mass fraction. (The Zeldovich mechanism gives the NOx formation rate rising exponentially with temperature.)
The reduction in NOx due to EGR was for a long time thought to be a result of the increase in the heat capacity of the charge caused by the presence of recirculated CO2. However, recent work has shown that the reduction of oxygen in the inlet charge (dilution effect) is the dominant factor [ 57 ]. The work investigated the way that EGR influences diesel engine combustion and emissions. Findings from the investigations included the effect of CO2 dissociation (chemical effect) on exhaust emissions is small; the high heat absorbing capacity of CO2 (thermal effect) had only a small effect on exhaust emissions including NOx. The reduction in the inlet charge oxygen (dilution effect) is the dominant effect on emissions, resulting in very large reductions in exhaust NOx at the expense of higher particulate and unburned hydrocarbon emissions and lower engine output and fuel economy.
Tropospheric ozone interacts with weather and climate
2.3 Lightning NOx emission
Renewable Energy Policies and Barriers
4.1 Renewable Energy Set-Asides
To meet National Ambient Air Quality Standards, the U.S. Environmental Protection Agency requires 22 U.S. states and the District of Columbia to reduce NOx emissions significantly by 2007. States can meet emission reduction targets through actual emission reductions or purchase of emission reduction credits from other states participating in a region-wide NOx trading program. States can allocate, or set-aside, a percentage of the total state NOx allowances to energy efficiency and renewable energy. Eligible renewable energy producers receive these set-aside allowances and can sell them to fossil-fuel-based electricity generators to enable those generators to stay within their NOx allocation. The additional revenue from sales of these set-aside allowances can potentially provide stimulus for renewable energy development, although to date few states have implemented renewable energy set-asides.
Nitrogen Cycle, Atmospheric
II.C.2 Acid Rain
Acid precipitation, or acid rain, can causes significant impacts on freshwater, coastal, and forested ecosystems. Both NO3 − (from NOx emissions ) and SO 4 2− (from SO2 emissions) contribute significantly to acid rain. The relative ratio of SO4 2− / NO3 − in precipitation will be substantially determined by the regional emissions of SO2 and NOx. In regions that get most of their energy from coal and other high-sulfur fuels, there will be significant emissions of SO2 unless scrubber technology is employed. Due to declining emissions of SO2 in developed regions of the world and increasing NOx emissions, from automobiles, the relative contribution of NO3 − is changing, with NO3 − contributing an increasing fraction of the acidity in acid rain. In ice cores collected in remote regions of the northern hemisphere, SO4 2− and NO3 − concentrations have increased significantly in the past 50 years, reflecting the large increase in source emissions due to anthropogenic sources.
How to reduce nox emissions
Reduction of NOx emissions
Click on «+» to develop chapter
Definitions and main sources
Molecular nitrogen (N2) is an inert gas that makes up about 80% of the atmosphere and is not dangerous for humans or the environment. Nitrogen, however, can react with oxygen to build nitrogen oxides, also called NOx, like nitrogen oxide (NO), nitrogen dioxide (NO2) and higher orders.
The generic term NOx refers to the sum of nitrogen oxide (NO) and nitrogen dioxide (NO2), expressed as NO2. Nitrous oxide (N2O), a greenhouse gas, is not covered in NOx. The main source for NOx is combustion where primarily NO is formed. NO is then rapidly converted to NO2.
N2O or laughing gas is a nitrogen oxide that is often emitted from natural sources and not always accounted to NOx due to its chemical structure. N2O is sometimes, but not everywhere, subjected to regulations for technical combustion. It can be emitted by fluidised bed combustion and other industrial processes.
NOx emissions contribute to acidification via formation of nitrous acid (HNO2) and nitric acid (HNO3), to eutrophication, to tropospheric ozone formation and (in particular NO2) to irritation and damage to respiratory organs. Furthermore, NOx may react with ammonia to form secondary fine particles with negative health effects.
Formation process in combustion
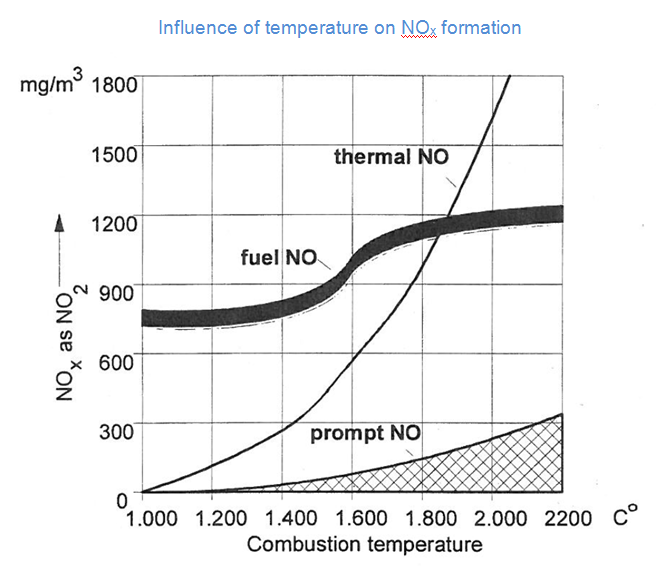
Nitrogen oxides are formed by three different mechanisms during combustions. The first mechanism – the thermal formation of NOx – usually accounts for the biggest share of NOx in combustion processes with high temperatures, like the combustion of gaseous or liquid fuels. If the peak flame temperature is below 1000°C the fuel NOx mechanism accounts for the majority of the NOx emissions.
The relationship between the combustion temperature and the NOx-formation of each path is shown in teh following table. While the positive correlation of fuel- and prompt NOx with temperature is about linear, the amount of thermal NOx is rising disproportionately fast with increasing combustion temperature.
In rich fuel-air ratios, less oxygen is available to react with the N2 in the combustion air and the fuel bound nitrogen. Therefore, both the thermal NO and fuel NO are reduced. The amount of prompt NOx, however, can be expected to rise (cf. Figure above).
The effect of the retention time also depends on the fuel-air ratio. A long retention time in rich air mixtures can reduce emissions due to the decomposition of NOx. In lean fuel-air ratios and high temperatures, however, new thermal NOx will be formed.
General approaches to reduce NOx emissions from combustion sources
In order to reduce NOx formation and NOx emissions from combustion processes different types of measures like energy efficiency improvements, fuel switch as well as primary and secondary measures are applied. To achieve the most efficient NOx reduction, beyond energy management measures, a combination of measures should be considered. To identify the best combination of measures, a site-specific evaluation is needed.
Primary measures gain to reduce the production of NOx during combustion, while secondary measures reduce the amount of already formed NOx in the flue gas. Not all measures are equally well-suited for all combustion techniques.
Fuel switching
Switching to low NOx producing fuels is one option to reduce NOx emissions but is governed by country specific conditions such as infrastructure and energy policy. Fuels with high nitrogen content like heavy fuel oil and coal may lead to high fuel NOx formation and hydrogen rich fuels like natural gas as a result of high combustion temperatures to high thermal NOx formation. The choice of the fuel may also have effects on other emissions like sulphur, particulate matter and greenhouse gas emissions as well as on applicability and need of abatement measures.
Fuel cleaning
Fuel cleaning to remove nitrogen is not a commercial option. Hydroprocessing in refineries, however, also reduces the nitrogen content of end products.
Primary Measures
Primary measures avoid or reduce the formation of NOx according to the three mechanisms described in the section ‘Formation’. Primary measures reduce NOx generation at the source by a number of different principles or methods or a combination of them:
In the following paragraphs, an overview on available primary measures is given. Their applicability depends on the industrial sector and the production process.
Reducing peak temperature
As thermal NOx formation depends largely on the combustion temperature, a reduction in temperature is one option to reduce NOx formation. Reducing peak temperature can be achieved by the following methods i) diluting the heat produced during the combustion process, ii) cooling down, and iii) reducing the oxygen available for combustion but also by applying other combustion techniques like fluidized bed combustion (FBC) which operates at lower temperatures and includes an inherent air-staging. Main methods for reducing peak temperature are:
Reducing residence time at peak temperature
As thermal NOx formation depends largely on the time the fuel gas remains in the high temperature region, reducing this residence time reduces also NOx formation. Methods to reduce residence time include:
Chemical reduction of NOx during combustion process
NOx can be reduced to N2 using a reducing agent which is itself oxidized. The principle of chemical reduction is widely used in secondary measures but can be also used as a primary measure when reduction takes already place during the combustion process. Main methods are:
Reducing nitrogen in the combustion process
Reducing NOx formation by reducing the available nitrogen can be achieved using nitrogen poor fuels like natural gas (see fuel switch) as well as by using oxygen instead of air for the combustion process.
The following primary measures which are based on the principles and methods described above are mainly in use, each with its specific advantages and disadvantages.
Some of the primary measures are typical for retrofit, others for new installations and others are only applicable in new installations.
The techniques reported above are an inventory of available technologies to reduce NOx emissions, which does not mean that those reported technologies are applicable to each industrial sector or process of production.
Modern combustion plants usually implement a combination of different primary measures in order to reduce emissions and ensure a high efficiency.
Secondary Measures
Secondary measures or so-called end-of-pipe techniques reduce the emission of pollutants without modifying the combustion process itself. This can be obtained by converting pollutants into non-harmful products or by precipitating pollutants from the flue gas before emitting it into the environment. Modern combustions plants are often equipped with several secondary measures (dust precipitator, flue-gas desulfurization, etc.) to reduce the contents of different pollutants. The retrofitting of secondary measures usually requires additional space for utilities. Supplementary fans and heaters might be required to overcome the increased pressure drop and obtain the necessary flue gas temperatures.
Selective Catalytic Reduction (SCR)
Selective Catalytic Reduction (SCR) is typically used for boilers between 25 and 800MW and large gas turbines and can achieve reduction rates above 90% (EPA, 2002a). In an SCR, NOx is reduced chemically by reaction with ammonia or urea in a catalytic bed. An aqueous solution of ammonia or urea is injected in the flue gas and reacts at the catalyst surface according to the following equations:
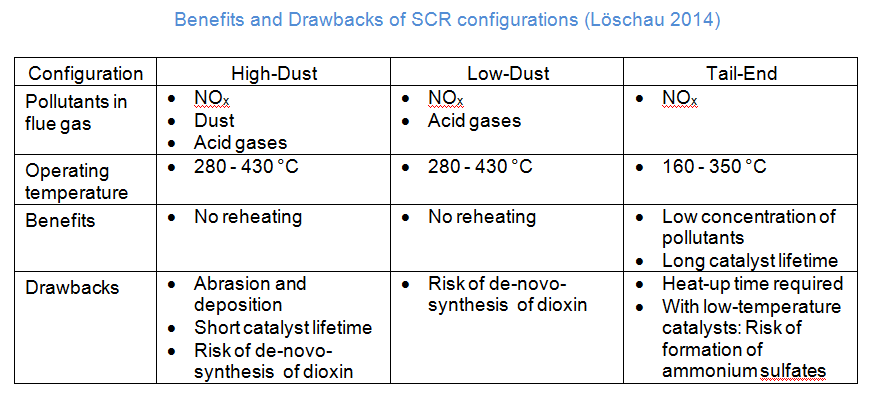
Base metal oxides, zeolites, iron oxides or activated carbon are used as catalysts. The different catalysts work at different (flue gas) temperatures between 170-510°C. The selection of the catalyst is influenced by the position of the SCR in the flue gas processing path and the presence of other pollutants in the flue gas. There are three different configurations for the integration of an SCR reactor in the flue gas treatment system (European Comission, 2013).
Configurations
The three possible configurations are shown in the following figure. The SCR is installed directly downstream the combustion and upstream the dust precipitator in the high-dust configuration. Therefore, flue-gas reheating is not necessary and the operating costs of the installation are kept relatively low. Modern catalysts have an improved resistance against abrasion and catalyst poisons. Catalyst poisons are, however, still the biggest drawback for this configuration. To overcome the drawbacks of the high-dust configuration and enhance the lifetime of the catalyst, the SCR can be installed downstream the dust precipitator in a low-dust configuration. To obtain sufficient temperatures in the SCR, special high-temperature dust-precipitators have to be installed. This can make a low-dust configuration an expensive option for retrofitting, since the dust precipitator might have to be replaced, too. The tail-end configuration, however, can be a favourable solution for existing plants. In this case, the SCR is installed as the last secondary measure downstream the dust precipitator and the flue-gas desulfurization. Compared to the other configurations, the flue gas is very clean at its entry into the SCR system and the danger of abrasion and catalyst poisoning is lower. Hence, a smaller catalyst may be sufficient, lowering the total investment. Yet, a drawback can be the necessary reheating of the flue gas. For an activated coal catalyst, a heat exchange of the raw gas with the clean gas might be sufficient, while other catalysts may require additional burners to reheat the flue gas.
Reduction Rate: 80-95%
Suitability: All fuels and combustion processes. For gas turbines a constant NH3/NOx ratio is more difficult to obtain. The ammonia slip will be higher (5-10 ppm) in order to obtain a high NOx reduction (95%).
Other Effects: Ammonia slip must be avoided due to legal regulations in many countries, but also because fly ash cannot be sold if the ammonia content exceeds a certain level (usually 2 ppm).
Installing an SCR allows to run the boiler or engine with higher NOx formation during combustion, achieving an up to 5% higher efficiency.
Parameters to be considered for technical reason
The selection of the most suitable measure depends on many factors related to e.g.:
The following table gives a brief overview about the performance of primary and secondary measures for reducing NOx emissions in large combustion plants; cf. the sectoral chapters for more detailed information on sector specific issues.
Information provided by industry and accepted by the Clearing House Evaluation Committee
This section presents technological developments in reduction techniques. The documents presented were submitted to the Clearing House Committee (CHEC) by industrial or state organisations or industrial companies. There are published after agreement of the CHEC.
NOx abatement primary measures
The following document was submitted by Babcock Wanson on 30 March 2017. The CHEC agreed to publish the document on 19 October 2017.
This document addresses the technical characteristics of a primary NOx abatement technique for combustion installations using gaseous fuels. Claimed performances are 80 mg/Nm3 et 3% O2.
The following documents were submitted by Fives Pillard in February 2017. The CHEC agreed to publish these documents on 19 October 2017.
The documents address the technical characteristics of a primary NOx abatement technique (ultra low NOx burner) for combustion installations using gaseous fuels. The claimed performances are below 50 mg/Nm3 at 3% O2. The documents present performances obtained in a chemical combustion plant of 8 MW, in a 15 MW water tube boiler, in a water tube boiler of 17 MW.
How to Reduce NOx: A Guide to Low Emission Boilers
Home » Blog » How to Reduce NOx: A Guide to Low Emission Boilers
Across the United States, there are over 60,000 industrial boilers, and virtually everyone wants to know how to reduce NOx. Whether you have a large utility boiler or industrial boilers, it’s important to have a significant focus on NOx reduction. Continue reading to learn more about innovative ways of how to reduce NOx.
How Is Nitrogen Oxide NOx Produced by Boilers?
Before delving into how to reduce NOx, it’s important to have an understanding of how nitrogen oxide is produced. Boiler burners use combustion to produce heat to make hot water or steam. In the process, nitrous oxide or NOx is produced as a byproduct of combustion. This pollutant can have harmful effects on the environment and is a large contributor to particulate matter, ozone, and acid rain.
In general, NOx has three main sources:
The majority of NOx emissions are the result of nitrogen in the fuels used or from nitrogen in the combustion air, which is thermal NOx. For oil-fired boilers and natural gas-fired boilers, thermal NOx comprises most NOx produced by industrial boilers.
Thermal NOx emissions will increase as peak flames temperatures increase. At the same time, NOx emissions are affected by the availability of oxygen in the boiler combustion zone.
How to Reduce NOx
When you are considering how to reduce NOx emissions, the most important step is to choose a qualified consultant, service provider, or vendor. This expert will help you conduct a boiler characterization and choose the right boiler or NOx reduction measure. However, the most common methods taken to reduce NOx are listed below.
Low NOx & Ultra Low NOx Burners
Undoubtedly, one of the most effective ways to reduce NOx is through Low NOx and Ultra Low NOx burners. Low NOx burners can reduce your NOx emissions to 30 pm. This equipment uses FGR and can maintain 3 to 5% excess O2 with strong controls. Low NOx burners offer good flame stability and turndowns that are less than the ratio of 8 to 1.
With Ultra Low NOx burners, you can achieve emissions anywhere between 7 to 15 ppm. These burners use adjusted fuel/air ratios, FGR, as well as staging to achieve exceptionally low emissions. Some Ultra Low NOx burner designs may have:
The Ultra Low NOx technology is continually improving with more conventional designs aimed at achieving greater efficiency.
Burner System Modifications and Tune-Ups
If you are not in the market for a new Low or Ultra Low Nox burner, the most prudent NOx reduction measures will start with a boiler tune-up. This maintenance solution includes the verification and optimization of the current operating parameters of the boiler.
By fine tuning the fuel content and oxygen in the combustion zone, you can reduce NOx emissions and improve efficiency. After performing simple tune-ups, systems can be utilized to automatically trim and monitor fuels and oxygen for optimum performance.
However, it’s imperative any and all of these procedures are performed by a qualified and factory-trained service professional. Failure to do so can result in serious hazards and injuries.
Burners out of Service
Burners out of Service or BOOS is another technique for how to reduce NOx. This technique can be used for boilers with multiple burners. It includes taking one or more than one of the burners out of service, which reduces the peak flame temperature. As a result, NOx emissions can be significantly reduced by several mechanisms of combustion.
Boiler De-Rating
In some cases, a facility’s steam needs and demands drop. The changing needs could be because of the implementation of more energy efficient processes or because of phased-out equipment. In either case, a boiler can be de-rated, which means it will fire at lower loads. Under these conditions, NOx emissions will be reduced by effectively lowering the firing rate.
Low Excess Air
For boilers that use spud or ring burners, a number of modifications can be made to:
These measures are effective at reducing NOx emissions. In some instances, air registers around the burner can be adjusted to help minimize NOx emissions.
Stage Combustion
Typically, stage combustion is only achievable in boilers with large furnace volumes. Stage combustion promotes fuel and air reactions in multiple stages or zones instead of all at once. As a result, stage combustion produces combustion properties, such as lower peak flame temperatures, that are much more conducive for low NOX emissions.
Contact Applied Technologies of New York
At Applied Technologies of New York, we help educational facilities, pharmaceutical firms, multi-residential apartments, as well as hospitals and medical facilities achieve greater efficiency and performance. We partner with the top boiler and combustion related manufacturers in the world. Simply put, we will help your facility learn how to reduce NOx and achieve greater performance out of your equipment.
Contact Applied Technologies of New York today for an innovative and cost-effective solution.
The whats, hows and whys of NOx emissions in commercial boilers
NOx emissions are the ‘hot topic’ of the industry. In this article, we investigate why they are a reason for concern, how they are generated and what we can do to reduce them.
What are NOx emissions?
NOx is a collective term that stands for nitrogen oxides which mainly consist of nitric oxide (NO) or nitrogen dioxide gas (NO2).
In the presence of sunlight, NOx emissions can produce ozone (O3). Since an oxygen molecule (O2) is stable by sharing four electrons (two per atom), creating a covalent bond, the presence of a third oxygen atom in the bond in ozone (O3) makes it unstable and hence more reactive/toxic than oxygen. This can have harmful effects on throat and lungs (including aggravation of asthma and emphysema) but also irritate eyes and mucous membranes.
Why should we be worried about NOx emissions?
Nitrogen oxides themselves mostly affect the lungs, reducing immunity to lung infections. It can also aggravate symptoms of asthma, increasing the intensity and quantity of attacks, making it an even bigger problem for children with the condition.
Where do NOx emissions come from?
The main source of NOx emissions in the modern world is the combustion of fossil fuel such as by car engines and boilers.
How does commercial heating produce NOx emissions?
In a commercial boiler, fuel such as natural gas is ignited which is used to heat water to be distributed in the heating system.
In theory, this involves the burning of only methane (CH4 – main component of natural gas) and oxygen (O2 – injected air) which produce carbon dioxide (CO2) and water (H2O).
However, in reality, air and natural gas are made up of several elements:
As a result, there are many by-products that are created during combustion. With nitrogen being very common in the mix, both nitric oxide (NO) and nitrogen dioxide (NO2) are just two of them.
What affects the production of NOx emissions in commercial gas boilers?
There are different types of NOx formation in combustion (thermal, prompt, and fuel) but for condensing gas boilers, thermal NOx is the most important one.
For a fully premixed burner, NOx emissions are fundamentally correlated to the flame temperature and depend on the following factors;
Air dilution λ value
Higher λ values lower the flame temperature and reduce the NOx emission.
Burner & gas air ratio controls
Further advances in burners and combustion controls now permit combustion in leaner conditions (optimised mix of air and fuel) which can improve NOx emissions.
Modulating characteristic
Since the heat requirements for a boiler do not stay the same over the entire year due to changing seasons, it might just be running at 20% of its capacity (low modulation) when it is warmer or 80% (high modulation) when it gets colder. A boiler firing at a lower rate has lower NOx emissions compared to firing at full capacity. For this reason, the NOx value assessed according to BS EN15502 is a weighted average taken from the boiler operating at different loads.
The result is then converted back to a dry air figure (no excess air present). Without the conversion (= with excess air), the result is not comparable due to favourable conditions giving a ‘better’ result (= lower NOx emissions). The dry air figures ensure a level playing field to compare manufacturers’ NOx figures.
Efficient heat transfer
The lowest flame temperature will be achieved by enabling the most efficient heat transfer away from the combustion chamber.
Gas group
The gas type will influence the flame temperature by relation to its calorific value (CV). Higher CV’s create a higher intensity flame and higher NOx.
These measures are all applicable to conventional premixed steel, metal fibre and ceramic burner types, in combination with a well-designed gas air ratio control system. This will enable NOx emission levels sufficient for the next generation domestic and small commercial boilers.
A trade-off between efficiency and NOx?
Taking our Upton modular boiler as an example, the gas air mixture is premixed before entering the burner which ensures the combustion gas is fully mixed. The burner has a metal fibre surface which distributes the combustion gas evenly over the surface of the burner. This, in combination with a high efficiency condensing heat exchanger, helps to keep the combustion temperature low and inhibits the formation of thermal NOx.
While this burner/heat exchanger design helps to cut NOx emissions up to a certain point, lowering the combustion temperature further can reduce the seasonal efficiency of the boiler. However, Ecodesign and CO2 budgets have been the main driver to increase seasonal efficiency to reduce carbon emissions. As a result, there would be a trade-off in efficiency to achieve substantially lower NOx levels.
Is there a standardised way of measuring NOx emissions from commercial heating and publishing NOx figures?
BS EN 15502 gives ways of measuring NOx. All boilers under 1000kW should adopt this methodology. This is a weighting formula to create an annualised emission and corrects to reference conditions.
Modulating boilers adjust their fire/gas use according to the heat load, making them more efficient and ‘clean’. Emission samples are hence taken across the entire modulation range when the boilers are working at lower or higher fire (i. e. spring, summer, autumn and winter heat loads) and then adjusted to reference conditions of 0% O2 (dry air free).
If the boiler is under 70kW, the manufacturer’s product fiche should have a section detailing emissions, this would be in line with this methodology. This should be checked against published data as this could be quoting either wet NOx levels (not dry air free) or levels at minimum modulation.
All of the published data of Hamworthy products is defined in this way (0% excess oxygen, dry air). We must be careful when analysing different manufacturer’s products to ensure we are comparing apples for apples. By using the British Standard we can ensure accurate comparisons are made.
Is there any legislation to reduce NOx emissions in commercial boilers?
Ecodesign
The latest limits from Ecodesign in September 2018 reduced the emission level on all natural gas boilers up to and including 400kW to a level of 56mg/kW.
This changed from net figures to gross. The previous best classification was Class 5 and stated at 70mg/kWh net. 56mg/kWh gross converted is 62mg/kWh net, so a reduction has been made. BS EN 15502 gives guidance on how these measurements are done and a new Class 6 was set up to comply with the Energy-related products (Ecodesign) requirement.
Medium Combustion Plant Directive
Since 20th December 2018, medium combustion plants (excluding plants and gas turbines) for new buildings, which commercial boilers fall under, with a thermal input between 1MW to 50MW must stay below NOx emissions of 100mg/NM³ (gas) or 200mg/Nm³ (oil). All of Hamworthy’s gas/air premix boilers are below these levels and have been tested in accordance with this standard.
What else is being done to reduce NOx emissions from commercial heating?
London, which regularly breaches air pollution limits for the entire year and where commercial gas consumption produces 7% of its NOx emissions, has the power to set its own limits. As a result, schemes for natural gas boilers with maximum NOx levels that vary from 24mg/kWh to 40mg/kWh are now being specified.
In July 2018, London’s Mayor Sadiq Khan launched the Cleaner Heat Cashback scheme to improve air quality. This programme was supposed to incentivise London’s SMEs to replace their inefficient boilers for which they could claim a part of the expenses back. Due to low demand, the funding for the scheme was significantly reduced but stays open for applications until March 2020.
BREEAM
BREEAM is an assessment method for the environmental impact of a building. BREEAM credits are awarded if a building meets certain criteria in terms of acoustics, renewables, floods managements or commercial boilers (pollution/local air quality) etc.
While it is non-mandatory, there is a large uptake in London.
There are currently 3 BREEAM schemes in use:
BREEAM 2014 is still applied for any new construction started pre 2018.
This scheme awards:
BREEAM 2018 applies to any new construction post 2018.
This scheme awards:
In terms of product development, we are always exploring how we can improve our products. As part of this process, we are looking into burners and combustion controls which permit leaner conditions – using less fuel and improving efficiency to investigate and adopt lower NOx emission targets where achievable.
In operational terms, there is already a lot that a user of commercial boilers can do to reduce NOx and carbon emissions. As outlined above, if a boiler is modulated down (lower fire), this improves efficiency and also decreases NOx emissions. Effective use of built-in boiler controls and measures such as weather compensation can reduce emissions. We talk more about the benefits of boiler controls in this article.
NOx Emission Reduction Strategies
by Marc Karell and Amit Chattopadhyay
The reduction of NOx emissions is a major goal of the Clean Air Act Amendments because of their known role in the formation of ground-level ozone. The U.S. EPA believes that facilities can reduce NOx emissions from their most common sources, combustion equipment, using reasonable means.
Many NOx-control technologies have been successfully applied to stationary combustion sources. Selection of an appropriate technique will depend on the type of facility (for example, industrial boiler, gas turbine, municipal-waste combustor), site-specific conditions, and regulatory and economic considerations.
NOx Formation
NOx is formed by two primary mechanisms, resulting in thermal NOx and fuel-bound NOx. A third mechanism, «prompt NOx» accounts for a minor share of NOx formation.
Thermal NOx formation occurs only at high flame temperatures, when dissociated nitrogen from combustion air combines with oxygen atoms to produce oxides of nitrogen such as NO and NO2. Formation of thermal NOx increases exponentially with combustion temperature, and increases as a function of the square root of the quantity of oxygen in the combustion zone.
Fuel-bound NOx formation is not limited to high temperatures, but is dependent upon the nitrogen content of the fuel.
The best way to minimize NOx formation is to reduce flame temperature, reduce excess oxygen, and/or to burn low nitrogen-containing fuels.
Available NOx Reduction Strategies and Technologies
The following represents proven, available NOx-reduction strategies and technologies for combustion sources.
Fuel switching. Fuel switching is the simplest and potentially the most economical way to reduce NOx emissions. Fuel-bound NOx formation is most effectively reduced by switching to a fuel with reduced nitrogen content. No. 6 fuel oil or another residual fuel, having relatively high nitrogen content, can be replaced with No. 2 fuel oil, another distillate oil, or natural gas (which is essentially nitrogen-free) to reduce NOx emissions.
Flue-gas recirculation (FGR). Flue gas recirculation involves extracting some of the flue gas from the stack and recirculating it with the combustion air supplied to the burners. The process, by diluting the combustion air with flue gas, reduces both the oxygen concentration at the burners and the temperature. Reductions in NOx emissions ranging from 30 to 60% have been achieved.
Low NOx burners. Installation of burners especially designed to limit NOx formation can reduce NOx emissions by up to 50%. Greater reduction efficiencies can be achieved by combining a low-NOx burner with FGR—though not additive of each of the reduction efficiencies. Low-NOx burners are designed to reduce the peak flame temperature by inducing recirculation zones, staging combustion zones, and reducing local oxygen concentrations.
Derating. Some industrial boilers can be derated to produce a reduced quantity of steam or hot water. Derating will decrease the flame temperature within the unit, reducing formation of thermal NOx. Derating can be accomplished by reducing the firing rate or by installing a permanent restriction, such as an orifice plate, in the fuel line.
Steam or water injection. Injecting a small amount of water or steam into the immediate vicinity of the flame will lower the flame temperature and reduce the local oxygen concentration. The result is to decrease the formation of thermal and fuel-bound NOx. Be advised that this process generally lowers the combustion efficiency of the unit by 1 to 2%.
Staged combustion. Either air or fuel injection can be staged, creating either a fuel-rich zone followed by an air-rich zone or an air-rich zone followed by a fuel-rich zone.
Staged combustion can be achieved by installing a low-NOx staged combustion burner, or the furnace can be retrofitted for staged combustion. NOx reductions of more than 40% have been demonstrated with staged combustion.
Fuel reburning. Staged combustion can be achieved through the process of fuel reburning by creating a gas-reburning zone above the primary combustion zone. In the gas-reburning zone, additional natural gas is injected, creating a fuel-rich region where hydrocarbon radicals react with NOx to form molecular nitrogen. Field evaluations of natural gas reburning (NGR) on several full-scale utility boilers have yielded NOx reductions ranging from 40 to 75%.
Reduced-oxygen concentration. Decreasing the excess air reduces the oxygen available in the combustion zone and lengthens the flame, resulting in a reduced heat-release rate per unit flame volume.
NOx emissions diminish in an approximately linear fashion with decreasing excess air. However, as excess air falls below a threshold value, combustion efficiency will decrease due to incomplete mixing, and CO emissions will increase.
The optimum excess-air value must be determined experimentally and will depend on the fuel and the combustion-system design. A feedback control system can be installed to monitor oxygen or combustibles levels in the flue gas and to adjust the combustion-air flow rate until the desired target is reached. Such a system can reduce NOx emissions by up to 50%.
Selective catalytic reduction (SCR). Selective catalytic reduction (SCR) is a post-formation NOx-control technology that uses a catalyst to facilitate a chemical reaction between NOx and ammonia to produce nitrogen and water.
An ammonia/air or ammonia/steam mixture is injected into the exhaust gas, which then passes through the catalyst where NOx is reduced. To optimize the reaction, the temperature of the exhaust gas must be in a certain range when it passes through the catalyst bed.
Typically, removal efficiencies greater than 80% can be achieved, regardless of the combustion process or fuel type used. Among its disadvantages, SCR requires additional space for the catalyst and reactor vessel, as well as an ammonia storage, distribution, and injection system. Also, a Risk Management Plan (RMP) in compliance with Federal Accidental Release Prevention rules may have to be prepared and submitted for ammonia storage.
Precise control of ammonia injection is critical. An inadequate amount of ammonia can result in unacceptable high NOx emission rates, whereas excess ammonia can lead to ammonia «slip,» or the venting of undesirable ammonia to the atmosphere.
Selective non-catalytic reduction (SNCR). Selective non-catalytic NOx reduction involves injection of a reducing agent—ammonia or urea—into the flue gas. The optimum injection temperature when using ammonia is 1850ºF, at which temperature 60% NOx removal can be approached. The optimum temperature range is wider when using urea.
Below the optimum temperature range, ammonia forms, and above, NOx emissions actually increase. The success of NOx removal depends not only on the injection temperature but also on the ability of the agent to mix sufficiently with flue gas.
Summary
Many facilities are being required to reduce NOx emissions as a result of legislation to limit emissions of precursors of ground-level ozone. Numerous options having varied success rates are available for NOx reduction and control. Therefore, careful thought must enter into the technical decision-making process.
As a general rule, reducing the flame temperature, reducing excess air, and/or burning low-nitrogen-containing fuels can minimize NOx formation. Post-formation control technologies are also available.
The various options must be reviewed in detail with respect to the level of NOx reduction necessary, the specific combustion source, the potential increase in emissions of other pollutants, site-specific constraints, and economic viability.
About the authors: Marc Karell and Amit Chattopadhyay are employed by Malcolm Pirnie, Inc. The former works at the company’s White Plains, NY, office and has more than 15 years of experience in the air-quality engineering field; the latter, at the Mahwah, NJ, office, has more than 25 years of experience in combustion engineering. Both are registered professional engineers.